Abstract
Background: FDA-approved chimeric antigen receptor T-cell (CAR-T) therapy are commonly administered in the inpatient setting given the average onset of cytokine release syndrome (CRS) is within the first 3 days post infusion. We and a few other centers have reported on the safety and feasibility of hospital-based outpatient practice (HBO) for CAR-T (Bansal et al, ASCO 2021). In Mayo Clinic Rochester practice, we have found the use of bridging therapy, elevated CRP and LDH as predictors for early hospital admission i.e., within 3 days post CAR-T infusion in patients with aggressive lymphoma (Bansal et al, ASCO 2021). Here, we report our experience with feasibility and safety of hospital-based outpatient (HBO) management of patients receiving the following CAR-T therapies: anti-BCMA CAR-Ts for multiple myeloma (MM), axicabtagene ciloleucel (axi-cel) for follicular lymphoma (FL), and brexucabtagene maraleucel (brexu-cel) for mantle cell lymphoma (MCL).
Methods: We retrospectively queried a prospectively maintained database of consecutive, adult patients with MCL, FL grade 1-3A (FL1-3A) and MM who received an FDA-approved CAR-T product between 3/2021 and 6/2022 at Mayo Clinic, Rochester. Clinical course from CAR-T infusion to the time that they were dismissed home from the treatment center was reviewed. To predict baseline risk factors for admission within 72 hours of infusion, a logistic regression model was fit. Variables were selected using stepwise AIC variable selection.
Results: Hospital based outpatient practice (HBO) is staffed by the same inpatient Immune Effector Cell care team, including nurses, advanced practice providers and physicians. These providers oversee patients in the hospital and HBO unit daily and provide continuity of care as patients transition between inpatient and outpatient care. HBO is the first point of contact 24/7 for patients and triage evaluations for direct inpatient admissions. The standard of care is to give lymphodepletion chemotherapy and CAR-T infusion on HBO and continue daily monitoring till day 7 and thereafter, as clinically needed until admission criteria are met. Fever, elevated CRP within 24-hour or less doubling time, or new neurologic symptoms are among the admission criteria utilized.
Among 50 CAR-T recipients, 39 (78%) pts were dosed outpatient, among whom 7 (18%) were not admitted (Table 1). Median length of hospital stay for the 32 patients who were admitted was 6 days (range, 2-49). Cytokine release syndrome (CRS) and immune cell associated neurologic syndrome (ICANS) incidences for the entire cohort were comparable to that reported in registration studies. There were 2 deaths due to CAR-T related toxicity within 30 days post infusion.
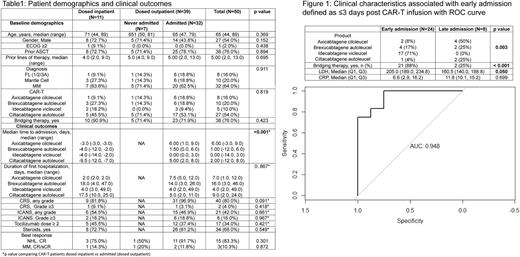
The median time to first admission from CAR-T infusion was 1 day (range 0-9), with 8 (25%) of admissions after 3 days. The most common cause of hospitalization was fever (25, 79%), of which 56% (14/25) were non-neutropenic. Work-up for fever was positive for infection in 4 (12.5%) patients. The median time to tocilizumab (26/32, 81%), steroid (1/31, 3%), escalation of oxygen support (4/32, 12.55), vasopressor (1/32, 3%), ventilator (1/32, 3%), hemodialysis (1/32, 3%) and ICU transfer (3/32, 9%) was 1 day (range, 0-31) after first admission. We applied the previous model (use of bridging therapy, elevated CRP and LDH) to this cohort and found this to remain correlated with early hospital admission (ROC AUC was 0.948, Figure 1).
Conclusion: Our expanded study in FL, MCL and MM confirms that HBO is feasible. Axi-cel, brexu-cel, Idecabtagene vicleucel and ciltacabtagene autoleucel can be safely dosed outpatient. Approximately 20% of the patients are never admitted. In addition, variables associated with aggressive disease appear to be applicable for prediction of early hospital admission in these new CAR-T products and indications. This could potentially be used to risk stratify patients for monitoring in outpatient CAR-T practices.
Disclosures
Dingli:Alexion Pharmaceuticals: Consultancy; Apellis Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; GlaxoSmithKline: Consultancy; Janssen Pharmaceuticals: Consultancy; Novartis: Consultancy; Takeda Pharmaceuticals: Consultancy; Sanofi S.A.: Consultancy. Kapoor:X4 Pharmaceuticals: Honoraria; Oncopeptides: Honoraria; Cellectar: Honoraria; GSK: Honoraria; Imedex: Honoraria; Pharmacyclics: Honoraria; Casma: Honoraria; Takeda: Research Funding; AbbVie: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Karyopharma: Research Funding; Loxo: Research Funding; Ichnos: Research Funding; Amgen: Research Funding; Regeneron: Research Funding; Sanofi: Honoraria, Research Funding. Wang:Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Loxo@Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; InnoCare: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Eli Lilly and Company: Membership on an entity's Board of Directors or advisory committees; Genmab: Research Funding; MorphoSys: Research Funding; Genentech: Research Funding. Kenderian:LEAH Labs: Current holder of stock options in a privately-held company, Research Funding; Lentigen: Research Funding; Humanigen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau; Morphosys: Research Funding; Tolero: Research Funding; Viracta/Sunesis: Research Funding; Life Engine: Current holder of stock options in a privately-held company; MustangBio: Patents & Royalties; Juno/BMS: Consultancy, Research Funding, Speakers Bureau; Kite/Gilead: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau; Mettaforge: Patents & Royalties. Kourelis:Novartis: Research Funding. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Shah:Astellas: Research Funding; Celgene: Research Funding; Marker Therapeutics: Research Funding. Ansell:SeaGen: Research Funding; Takeda: Research Funding; Bristol Myers Squibb: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Pfizer: Research Funding; ADC Therapeutics: Research Funding. Lin:Bluebird Bio: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Legend: Consultancy; Juno: Consultancy; Celgene: Consultancy, Research Funding; Novartis: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Gamida Cell: Consultancy; Takeda: Research Funding; Vineti: Consultancy; Sorrento: Consultancy; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal